肿瘤非同义突变与肿瘤新生抗原
在肿瘤起始,发展过程中,发生了成千上万的体细胞突变。其中大多数突变不稳定,不能获得生长优势,所以只是一过性的突变,称之为passenger mutations。少数突变可以诱导正常细胞恶变,促进肿瘤生长,对靶向药物产生耐受,称之为driver mutations。二者均可产生氨基酸改变,都称之为非同义突变(nonsynonymous mutations),产生和正常细胞不同的蛋白质。
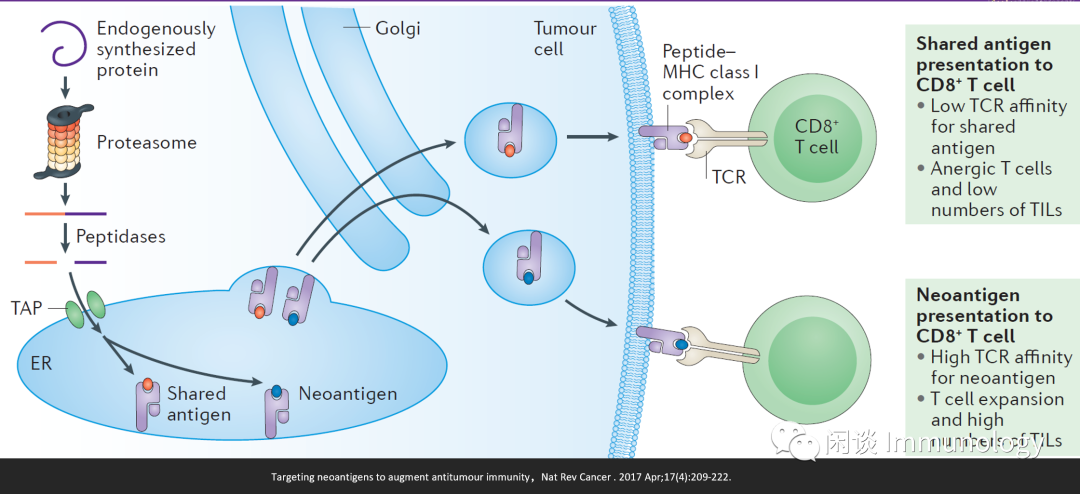
肿瘤新生抗原(neoantigens)主要来自于非同义突变。因为肿瘤新生抗原在正常细胞不表达,理论上可以刺激更强的T细胞反应 (高亲和力的TCR,T细胞扩增,和肿瘤组织浸润淋巴细胞数量增多,因而肿瘤新生抗原疫苗和免疫检查点联用效果好 ) 。
肿瘤新生抗原研究及临床应用简史
肿瘤新生抗原发现和验证流程
虽然对于肿瘤新生抗原有各种各样的质疑(准确性,异质性等),但是对这类只有肿瘤表达的抗原的深入认识,才能让我们更好的认识我们的敌人。
简而言之:健康细胞和肿瘤细胞进行DNA测序,发现肿瘤变异序列,进而RNA测序,通过生物信息学的方法预测蛋白酶体降解的序列,以及可被MHC分子递呈的序列。
最后一步,T细胞验证实验。
肿瘤新生抗原的应用
伴随诊断:肿瘤新生抗原的丰度,证实和多种抗肿瘤药(尤其免疫检查点抑制剂)疗效相关(争论见下文)。
治疗药物:
疫苗:个性化的肿瘤新生抗原肽,RNA,DC疫苗
细胞治疗:新生抗原特异性的T细胞治疗
临床应用的争论
开始有一些肿瘤医生提出:肿瘤新生抗原/肿瘤突变负荷(TMB)太多时,免疫检查点抑制剂疗效并不好。
当突变负荷增加到一定程度(窗口研究需要科学家们努力),会严重增加亚克隆的数量,导致极强的肿瘤异质性和肿瘤逃逸,产生差的临床结果。
参考文献
De Plaen E, Lurquin C, Van Pel A, Mariame B, Szikora JP, et al. 1988. Immunogenic (tum−) variants of mouse tumor cloning of the gene of tum− antigen P91A and identification of the tum− mutation. PNAS 85:2274–78
Wölfel T, Hauer M, Schneider J, Serrano M, Wölfel C, et al. 1995. A p16INK4a-insensitive CDK4 mutant targeted by cytolytic T lymphocytes in a human melanoma. Science 269:1281–84
Coulie PG, Lehmann F, Lethe B,Herman J, Lurquin C, et al. 1995.A mutated intron sequence codes for an antigenic peptide recognized by cytolytic T lymphocytes on a human melanoma. PNAS 92:7976–80
Robbins PF, El-Gamil M, Li YF, Kawakami Y, Loftus D, et al. 1996. A mutated beta-catenin gene encodes a melanoma-specific antigen recognized by tumor infiltrating lymphocytes. J. Exp. Med. 183:1185– 92
Novellino L, Renkvist N, Rini F, Mazzocchi A, Rivoltini L, et al. 2003. Identification of a mutated receptor-like protein tyrosine phosphatase kappa as a novel, class II HLA-restricted melanoma antigen. J. Immunol. 170:6363–70
Huang J, El-Gamil M, Dudley ME, Li YF, Rosenberg SA, Robbins PF. 2004. T cells associated with tumor regression recognize frameshifted products of the CDKN2A tumor suppressor gene locus and a mutated HLA class I gene product. J. Immunol. 172:6057–64
Brandle D, Brasseur F,Weynants P, Boon T,Van den Eynde B. 1996. A mutated HLA-A2 molecule recognized by autologous cytotoxic T lymphocytes on a human renal cell carcinoma. J. Exp. Med. 183:2501–8
Lennerz V, Fatho M, Gentilini C, Frye RA, Lifke A, et al. 2005. The response of autologous T cells to a human melanoma is dominated by mutated neoantigens. PNAS 102:16013–18
Ley TJ, Mardis ER, Ding L, Fulton B, McLellan MD, et al. 2008.DNA sequencing of a cytogenetically normal acute myeloid leukaemia genome. Nature 456:66–72
Matsushita H, Vesely MD, Koboldt DC, Rickert CG, Uppaluri R, et al. 2012. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature 482:400–4
Castle JC, Kreiter S, Diekmann J, Lower M, van de Roemer N, et al. 2012. Exploiting the mutanome for tumor vaccination. Cancer Res. 72:1081–91
Robbins PF, Lu YC, El-Gamil M, Li YF, Gross C, et al. 2013. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat. Med. 19:747–52
van Rooij N, van Buuren MM, Philips D, Velds A, Toebes M, et al. 2013. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J. Clin. Oncol. 31:e439–42
Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, et al. 2014. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature 515:577–81
Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, et al. 2014. Genetic basis for clinical response to CTLA-4 blockade in melanoma.N. Engl. J. Med. 371:2189–99
Tran E,Turcotte S, Gros A, Robbins PF, Lu YC, et al. 2014. Cancer immunotherapy based on mutationspecific CD4+ T cells in a patient with epithelial cancer. Science 344:641–45
Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, et al. 2015. Cancer immunology: Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348:124–28
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, et al. 2015. PD-1 blockade in tumors with mismatch-repair deficiency.N. Engl. J. Med. 372:2509–20
Carreno BM, Magrini V, Becker-Hapak M, Kaabinejadian S, Hundal J, et al. 2015. Cancer immunotherapy: A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science 348:803–8
Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, et al. 2017. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547:222–26
Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, et al. 2017. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 547:217–21 73.
Tran E, Robbins PF, Lu YC, Prickett TD, Gartner JJ, et al. 2016. T-cell transfer therapy targeting mutant KRAS in cancer.N. Engl. J. Med. 375:2255–62
McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, et al. 2016. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 351:1463–69
Bassani-Sternberg M, Braunlein E, Klar R, Engleitner T, Sinitcyn P, et al. 2016. Direct identification of clinically relevant neoepitopes presented on native human melanoma tissue by mass spectrometry. Nat. Commun. 7:13404
Ton N. Schumacher,Wouter Scheper,and Pia Kvistborg,Cancer Neoantigens,Annu. Rev. Immunol. 2019. 37:173–200
Mark Yarchoan et al,Targeting neoantigens to augment antitumour immunity,Nat Rev Cancer . 2017 Apr;17(4):209-222.
Anne Trinh, and Kornelia Polyak,Tumor Neoantigens: When Too Much of a Good Thing Is Bad,Cancer Cell 36, November 11, 2019